![[DIGITALE BIBLIOTHEK DER FES]](/images/digbib/d_digbib.gif)

SECTION of DOCUMENT:
[page number of print ed.: i]
OPENING ADDRESS
THE IMPACT OF BIOTECHNOLOGY ON AGRICULTURE AND HUMAN HEALTH
Opening speech by the Hon. K. Kangai, MP, Minister of Lands and Agriculture at the Research Council of Zimbabwe National Consultative Workshop on Impact of Biotechnology on agriculture and Human Health: Kadoma Ranch Hotel Conference Centre: 2-3 September 1999.
The Chairman of Biosafety Board of Zimbabwe
Dr Robbie Mupawose
Members of the Research Council of Zimbabwe
Members of the Biosafety Board
Distinguished Participants
Ladies and Gentlemen
I feel greatly honoured to have been asked to officially open this very important workshop on the Impact of biotechnology on Agriculture and Human Health in Zimbabwe.
The consultative workshop could not have come at a better time as Zimbabwe is expected to enunciate appropriate policies regarding this topical, issue of Genetic Engineering as it applies to agriculture, human health and the environment in Zimbabwe.
In the last 1000 years, science and technology has revolutionalised humanity significantly indeed. As we approach the dusk of one era and enter the dawn of a new millenium, it is fitting that we should stop briefly in our tracks and take stock of things.
It is perhaps in the field of agriculture and human health that the impact of science and technology has made a lasting impression. Of particular note is the significance of the science called biotechnology. I am reliably informed by our Zimbabwean scientists that it is through biotechnology that world agricultural food production has more than doubled and that food shortages and hunger in some parts of the world are more of the result of inequitable food distribution and not a result of a decline in world food production. The biotechnology of genetically modified organisms has resulted, for example in production of drought resistant and pest resistant cereals. It has also created tomatoes which have an enhanced flavour, enhanced texture and a long shelf-life. Milk production and yield has been dramatically increased by genetically modified cows. I am informed that a number of medicines are produced commercially in milk of genetically modified sheep, goats, cows and even rabbits. Insulin which is crucial for diabetic patients (and we have many diabetic people in Zimbabwe) is now produced in greater quantities from bacteria which have been modified biotechnologically into "human insulin producing organisms".
[page number of print ed.: 2]
There is no doubt that biotechnology has made a significant and positive impact on our lives. However, there are some ethical, moral and controversial concerns associated with the biotechnology of genetically modified organisms. The biotechnology of cloning and specifically human cloning raises concerns about the misuse of this science!
Cloning of human cells conjures up images of frightening speculation and imagination. Is it possible to experiment on human embryo without violating the embryo's freedom? Is it ethical, morally acceptable to tinker with human embryos? Of late, the safety of genetically modified agricultural produce has become questionable? Is it responsible or justifiable to expose people to genetically modified agricultural produce the long term safety of which, we are not very clear of! What are the long-term consequences of genetically transformed crops over the nontransformed crops?
It is with these questions and concerns in mind that the Research Council of Zimbabwe saw it prudent to hold this National consultative workshop.
Many nations have adopted legislation on the ethical and moral impact of biotechnology. I hope that at the end of this workshop, you will come up with recommendations which can be used to help government come up with a national policy on the biotechnology of genetically modified organisms and human cloning.
With these few words, it is my pleasure to declare this National Consultative Workshop officially opened and I wish you a fruitful two days of consultation.
Thank you.
[page number of print ed.: 3]
KEY NOTE ADDRESS
OVERVIEW OF RECOMBINANT DNA TECHNOLOGY
C.J. Chetsanga,
Director General, Scientific and Industrial Research and Development Centre,
P.O. Box 6640 Harare, Zimbabwe.
The advent of gene cloning technology has been made possible by the development of very powerful tools for selectively splicing those targeted sites on a DNA molecule at which one wishes to insert new genes. This selective splicing is carried out by DNA cutting enzymes called restriction endunucleases (REs).
1. Restriction Endonucleases
A large number of REs has now been identified. Each enzyme cleaves or cuts a specific site in a DNA molecule. The sites are called restriction sites or cleavage sites.
A DNA molecule is made up of the bases adenine (A), cytosine (C), guanine (G) and thymine (T). These bases form base pairs along the linear length of DNA molecule as:
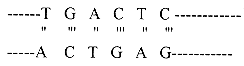
The A-T base has two hydrogen bonds, while G-C base pair has three hydrogen bonds.
The REs cut DNA along its lengths, and recognize the nucleotide motif at that site. I do not intend to give details of the molecular biology of the different REs and all the different cleavage sites that they recognize and cut, nor do I plan to give the names of the different bacterial species from which the REs have been obtained.
I shall give only one RE and the cleavage site that it recognizes:
EcoRI cleaves between G and A in the Sequence:
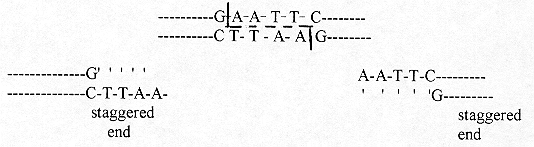
[page number of print ed.: 4]
The 2 staggered ends (cohesive ends) are called 'sticky' as they can readily come back together to recombine by similar sequences produced by the same EcoRI on the DNA of a suitable vector. A vector in this area of gene cloning is a piece of DNA from a plasmid or virus that also has a cleavage site of the configuration:

The viral or plasmid DNA is mostly circular
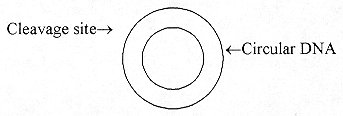
In gene cloning technology, one now gets a gene that they wish to clone and by the chemical synthesis, add the cohesive ends called synthetic linkers with similar base sequences.
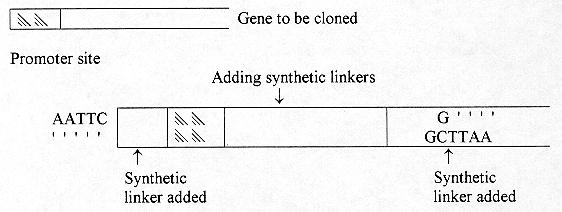
[page number of print ed.: 5]
2. Gene Cloning and Ligation
This gene is now ready to be cloned into the plasmid or viral, DNA
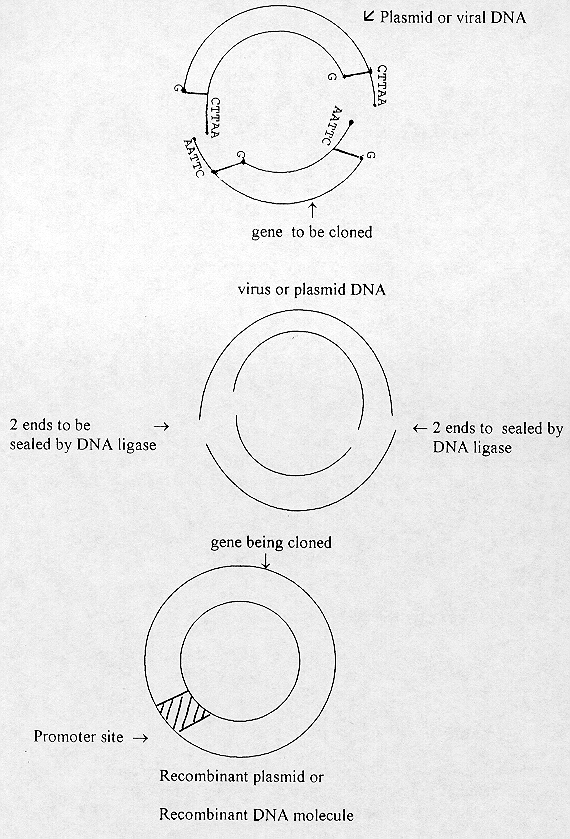
[page number of print ed.: 6]
The promoter site is a portion of the gene that can stimulate the expression of the gene in question.
The gene here could be a gene for making antibodies (vaccine) against the polio virus to prevent the poliomyelitis.
This DNA is now called recombinant plasmid DNA. The plasmid or virus is used as a vector which can now penetrate the particular bacterial species or baker's yeast cells can serve as a host for the plasmid or virus vector.
3. DNA Replication and Gene Expression in Host Cell
Inside the host cell, the recombinant DNA now replicates to form thousands of its copies. The host bacterial cells themselves will also be multiplying to form thousands of cells. During a certain stage in this process the genes in the vector DNA begin to be expressed. The expression of a gene means messenger RNA (mRNA) is made from that gene. In the vector DNA, the promoter of the polio vaccine gene will stimulate its expression so that multiple rounds of synthesis of the mRNA that codes for the polio vaccine is produced in large quantities.
4. mRNA Translation into Protein
Within the host bacterial or yeast cell, the mRNA will now be translated to make the protein vaccine for which it contains the genetic code. Yeast cells are more efficient system for making vaccines for eukaryotic gene systems.
The bacterial host cells can be grown in large fermentation tanks for industrial scale vaccine production. After overnight growth, the next day several kilograms of bacterial cells can be harvested. Each cell will have served as a bioreacter in which vaccine proteins will have been produced.
The harvested cells are lysed and the vaccine protein purified by established biochemical methods. The vaccine is then tested for its ability to evoke the appropriate immune response.
5. Vaccine for disease prevention
Biotechnology has been more effective in the production of pharmaceuticals and industrial products than it has achieved in the agricultural field.
Pharmaceutical products from biotechnology have been less controversial in society than genetically engineered food products.
The continuing streams of new generation recombinant vaccines are providing active competition to get to market by most of the major pharmaceutical houses. The list of new vaccines continue to grow and include Hepatitis B virus vaccine, peptide vaccines,
[page number of print ed.: 7]
anti-bacterial vaccines, among others. Vaccine action is often potentiated by adjuvants and immunostimulants. The production of monoclonal antibodies has been an important contribution to medicine.
A very active area of biomedical research has been in the production of biomolecules for therapy which include vaccines, interferons which prevent successful infective action and cytokinins which promote therapy by inducing cell divisions.
6. Diagnostics
The success of a physician in accurately diagnosing a disease is only as good as the support apparatus available to him for executing the diagnosis. Typically, they collect tissue, urine, faeces, or blood specimens and send them to laboratory for clinical analysis. Some classical diagnostic procedures are extremely reliable while others are very accurate.
The area of disease diagnosis is always going to remain a research frontier. The latest diagnostic tools are called DNA probes. These are nucleotide fragments that are complementary to a portion of the DNA that is under investigation. If we know the base sequences of the DNA of a bacterium like vibrio cholera, we can make a short nucleotide fragment (20-mer) that is complementary to a segment of V. cholera DNA.
Using the polymerase chain reaction, we can make more DNA molecules using the 20 nucleotide fragment as the primer. Whenever there is a cholera outbreak, we can use this short primer as a probe to determine if collected clinical specimens contain the V.cholera DNA sequences.
© Friedrich Ebert Stiftung | technical support | net edition fes-library | August 2001